Bartłomiej Żerek, Piotr Rózga
Adamed Sp. z o.o.
In the two past decades recombinant protein therapeutics revolutionized the countenance of the contemporary medicine and initiated effective therapies for numbers of refractory diseases. Since 1982, when the first therapeutic protein – recombinant human insulin – was introduced, the global market of biotechnology drugs has been still growing. In 2008 more than 130 molecules were approved for clinical use by FDA and at present there are almost 170 proteins and peptides in use.
The success of protein drugs was easy to predict. In comparison to small-molecule drugs protein therapeutics have several very important advantages:
• specific directing and higher efficacy with low number of side effects,
• better PK and PD,
• the specific interactions with the molecular target cannot be imitated by any chemical compounds,
• they are well tolerated and, as they are naturally produced by body, it is less probable that they elicit immune response,
• the clinical development and approval time can be shorter than in case of small-molecule drugs,
• their unique structure and functions allow the comprehensive patent protection,
• the recombinant DNA technology allows to choose the expression system (e.g. bacteria, yeast, insect cells, mammalian cells) dictated by the costs or the need of modification within the structure.
Actually the first life-saving insulin was purified from bovine and porcine pancreas in 1922. Though, the availability of the organs, the costs the production and the immunological reaction turned out to be significant problem on the industrial scale production and market development. Scarcely in the 70’s the recombinant DNA technology opened the new age of pharmaceutical biotechnology. For this reason, the definition of protein therapeutics covers drugs, which structurally are proteins or peptides, but also the fact that they are obtained with the recombinant DNA technology.
Today biopharmaceutics include: recombinant hormones, interferons, interleukins, hematopoietic growth factors, tumor necrosis factors, blood-clotting factors, thrombolytic drugs, enzymes, monoclonal antibodies (2nd generation) and vaccines.
The main applications are: diabetes, dwarfism, myocardial infarction, congestive heart failure, cerebral apoplexy, multiple sclerosis, neutropenia, thrombocytopenia, anaemia, hepatitis, rheumatoid arthritis, asthma, Crohn’s disease and cancers therapies.
The 1st generation of biopharmaceutics is dominated by structures based on the native sequences, identical with the proteins/peptides naturally occurring in the body tissues, whereas in the 2nd generation we find drugs with improved properties, especially PK, biodistribution, higher specificity, efficacy and minimized side effects. To achieve these desired features, the structures are deliberately modified by covalent attachment of the chemical compounds, introduction of certain changes within the sequences of the protein, fusions of two or more peptide chains or replacement of the sugar residues. The main challenge for the next, already 3rd generation of recombinant drugs, seem to be the new route of administration, new formulations and consequently even higher efficiency and safety.
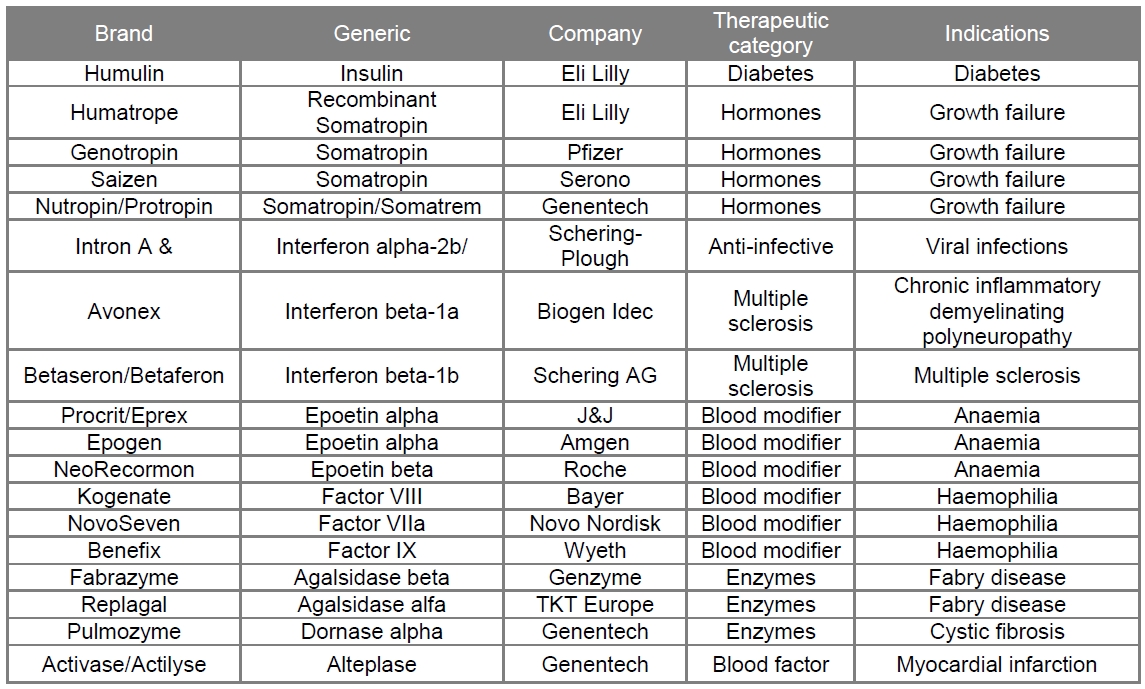
The first generation of therapeutic proteins
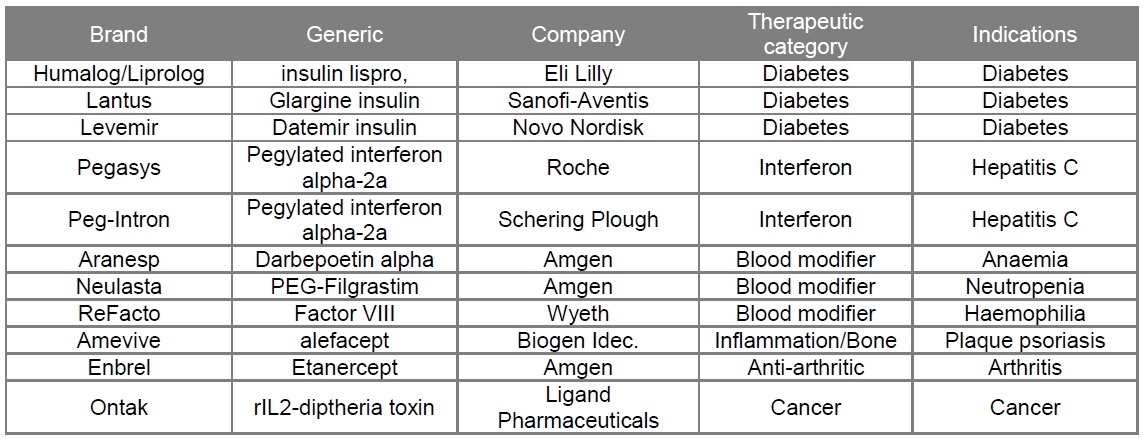
The second generation of therapeutic proteins
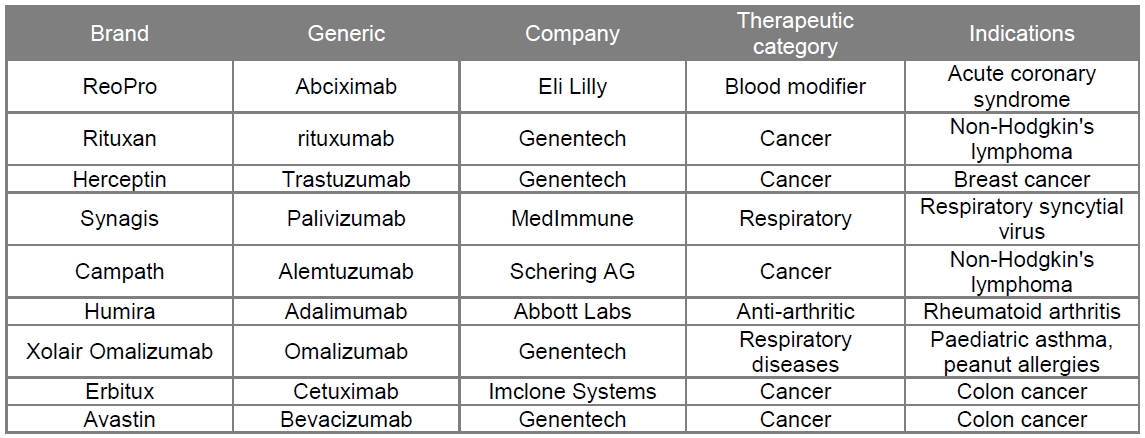
Monoclonal Antibodies
The drugs which are proteins or peptides are large and labile compounds that usually can be administered only by injection. There are several problems with the administration of proteins and peptides by injection. Firstly, the significant number of patients wants to avoid the pain of injection, especially during treatment of chronic diseases when regular and frequent injections are required. Secondly, injectable drugs might be expensive as they often have to be administered in a hospital or office setting. To avoid both of these problems many diabetics have been chronically self-administering insulin by injection. Traditional non-invasive delivery systems do not work for protein macromolecules as while pills or tablets enter the stomach the proteins are rapidly degraded by enzymes and hydrochloric acid. The oral administration of proteins and peptides is under research, but no such system is commercially available yet.
In 2003, the global market for therapeutic proteins was worth $37 billion, more by almost 19% than in the previous year, and achieved sales of over $90 billion in 2010. Much of this market is dominated by erythropoietins and monoclonal antibodies, which treat common diseases such as anaemia, arthritis and cancer. At the same time the global market for pharmaceuticals reached approximately $650 billion in the same year. Therefore, therapeutic proteins covered approximately 13.8% share of the global pharmaceutical market in 2010, a considerable proportion of the total.
How will the proteins market develop the near future? According to the GBI Research in the report “Therapeutic Proteins Market to 2017 - High Demand for Monoclonal Antibodies will Drive the Market” the market is likely to grow and to reach $141.5 billion in 2017. After Global Industry Analysts, Inc. in the “Protein Drugs – Global Market Report”, nearly 400 types of protein drugs are in the process of development and global market for protein drugs is estimated to reach $158.2 billion by 2015. However the expiration of the patents of key protein therapeutics on the one hand and budget limitations on the other will result in opening the market to biosimilar versions of first generation drugs. Finally Research and Markets in “Global Protein Therapeutics Market Forecast to 2015” states that the global market for biopharmaceutics is still growing and is likely to reach the level of $143.4 by 2016.
Market share of the Leading Therapy Classes, 2009

Market share of the Leading Therapy Classes, 2015 (estimated)
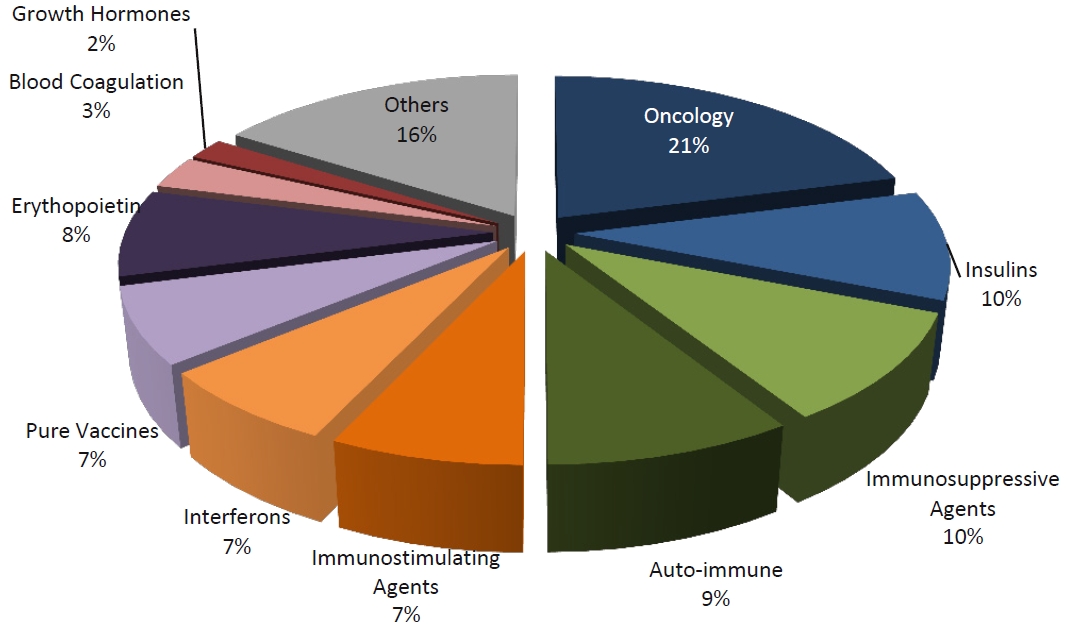
Without any doubts there is still a lot to be done in the protein recombinant drugs. Their development always means years of efforts and high risk. Though, even if only one is successful, the success will be outstanding.
Bibliography
1. Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008 Jan;7(1):21-39.
2. Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009 Mar-Apr;59(2):111-37. Review. Erratum in: CA Cancer J Clin. 2010 Jan-Feb;60(1):62.
3. Jarecka M., Borowicz P. Therapeutic and market expectancy of recombinant medicines. Biotechnologia. 2005 number: 4, 7-27
4. Werle M, Makhlof A, Takeuchi H. Oral protein delivery: a patent review of academic and industrial approaches. Recent Pat Drug Deliv Formul. 2009 Jun;3(2):94-104.
5. Martin P, Beyond the next generation of therapeutic proteins. BTi. 2006 October.
6. Global Protein Therapeutics Market Forecast to 2015 - Research and Markets 2012
7. Therapeutic Proteins Market to 2017 – High Demand for Monoclonal Antibodies will Drive the Market - GBI Research 2011
8. Protein Drugs – Global Market Report – Global Industry Analysts, Inc. 2010
9. Global Pharmaceutical Biotech Market 2010 – 2025 – visiongain 2009





